what group of organic compounds has a hydroxyl group -o-h attached to a carbon atom?
fourteen.two: Alcohols - Nomenclature and Classification
- Page ID
- 16042
Learning Objectives
- Place the full general structure for an alcohol.
- Identify the structural feature that classifies alcohols as primary, secondary, or tertiary.
- Name alcohols with both mutual names and IUPAC names
An alcohol is an organic chemical compound with a hydroxyl (OH) functional group on an aliphatic carbon cantlet. Because OH is the functional group of all alcohols, we often represent alcohols by the general formula ROH, where R is an alkyl group. Alcohols are common in nature. Most people are familiar with ethyl alcohol (ethanol), the active ingredient in alcoholic beverages, merely this compound is but one of a family of organic compounds known as alcohols. The family unit also includes such familiar substances every bit cholesterol and the carbohydrates. Methanol (CH3OH) and ethanol (CHthreeCH2OH) are the showtime two members of the homologous serial of alcohols.
Classification of Alcohols
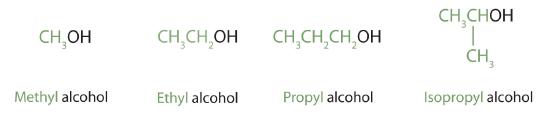
Alcohols with 1 to 4 carbon atoms are oftentimes called by mutual names, in which the name of the alkyl group is followed by the word alcohol:
According to the International Union of Pure and Applied Chemistry (IUPAC), alcohols are named past irresolute the ending of the parent alkane name to -ol. Hither are some bones IUPAC rules for naming alcohols:
- The longest continuous concatenation (LCC) of carbon atoms containing the OH group is taken as the parent compound—an methane series with the same number of carbon atoms. The chain is numbered from the end nearest the OH grouping.
- The number that indicates the position of the OH group is prefixed to the proper noun of the parent hydrocarbon, and the -eastward ending of the parent paraffin is replaced by the suffix -ol . (In circadian alcohols, the carbon atom bearing the OH group is designated C1, but the i is not used in the proper name.) Substituents are named and numbered equally in alkanes.
- If more than one OH group appears in the same molecule (polyhydroxy alcohols), suffixes such as -diol and -triol are used. In these cases, the -e catastrophe of the parent methane series is retained.
Figure \(\PageIndex{1}\) shows some examples of the application of these rules.
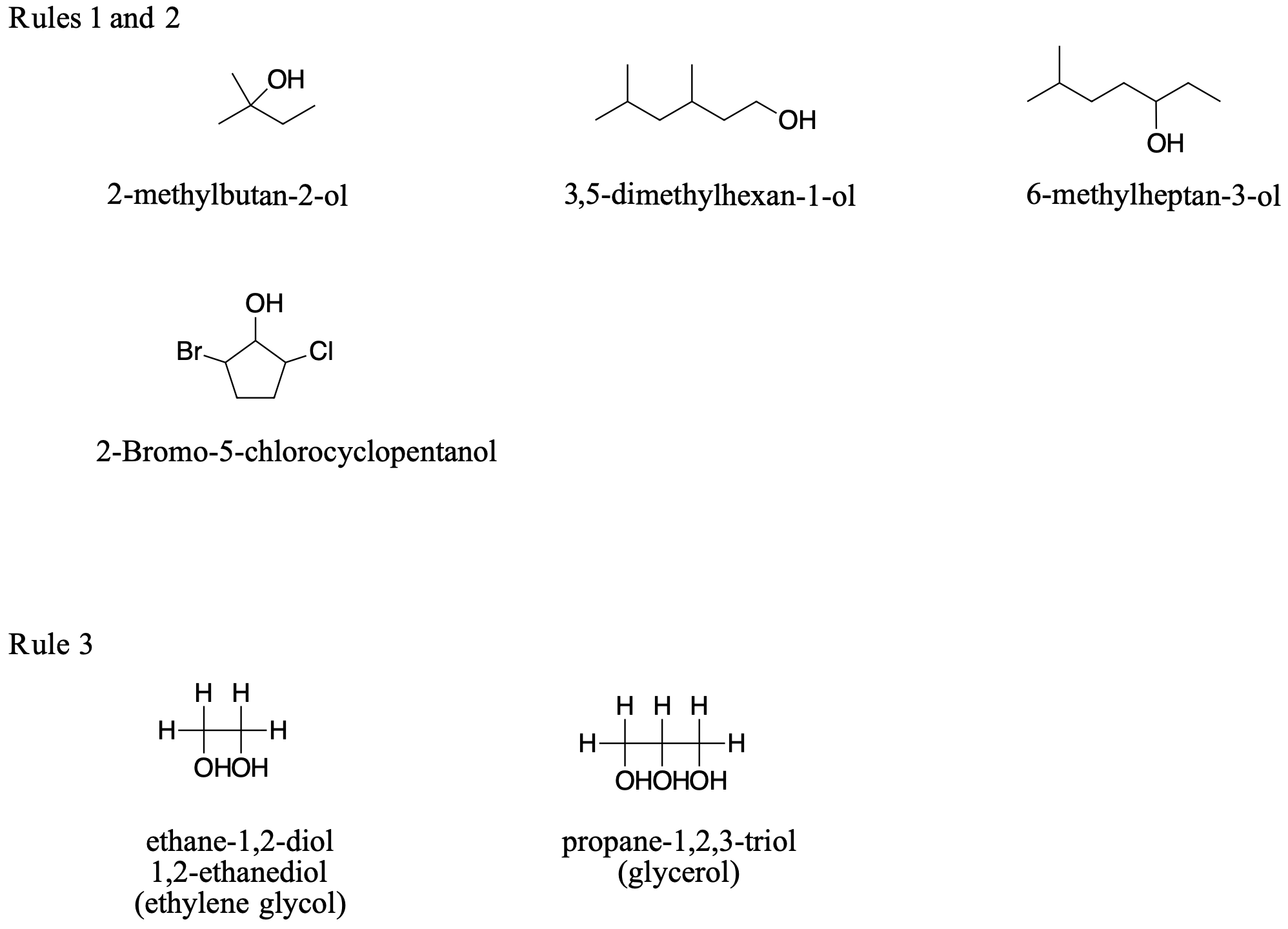
Figure \(\PageIndex{ane}\): IUPAC Rules for Alcohols. The names and structures of some alcohols demonstrate the use of IUPAC rules.
Instance \(\PageIndex{1}\)
Give the IUPAC name for each compound.
-
- HOCH2CHiiCH2CH2CH2OH
Solution
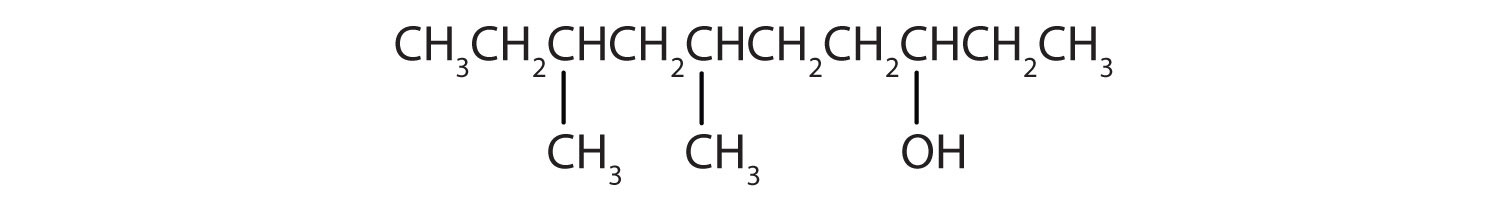
- Ten carbon atoms in the LCC makes the compound a derivative of decane (rule 1), and the OH on the third carbon atom makes it a 3-decanol (rule 2).
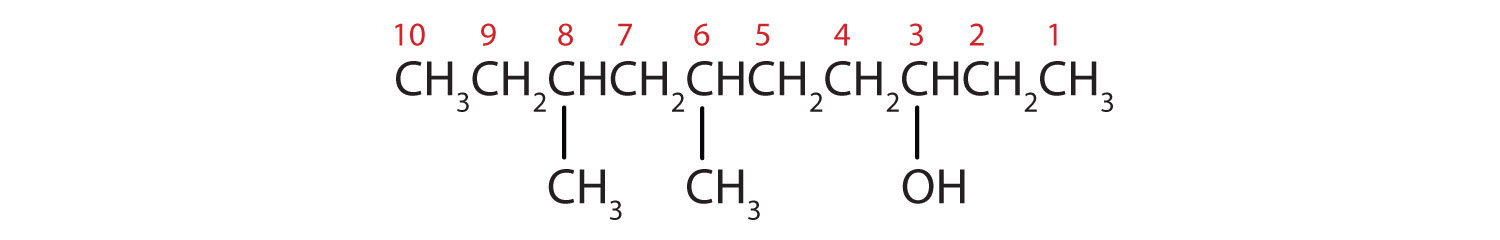
The carbon atoms are numbered from the cease closest to the OH group. That fixes the ii methyl (CH3) groups at the sixth and eighth positions. The name is half-dozen,8-dimethyl-3-decanol (not 3,five-dimethyl-8-decanol).
- Five carbon atoms in the LCC brand the compound a derivative of pentane. 2 OH groups on the first and fifth carbon atoms make the chemical compound a diol and give the name ane,5-pentanediol (rule 3).
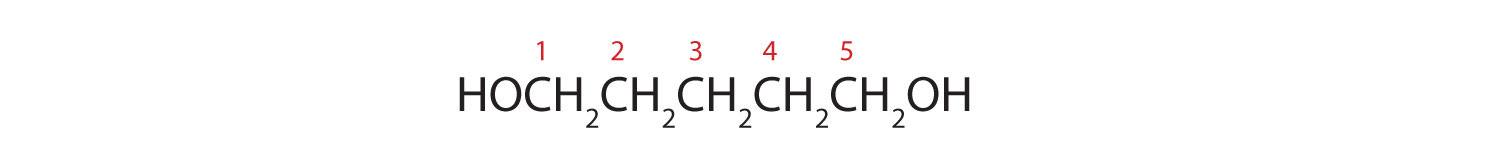
Exercise \(\PageIndex{one}\)
Give the IUPAC name for each compound.
Example \(\PageIndex{2}\)
Draw the construction for each compound.
- ii-hexanol
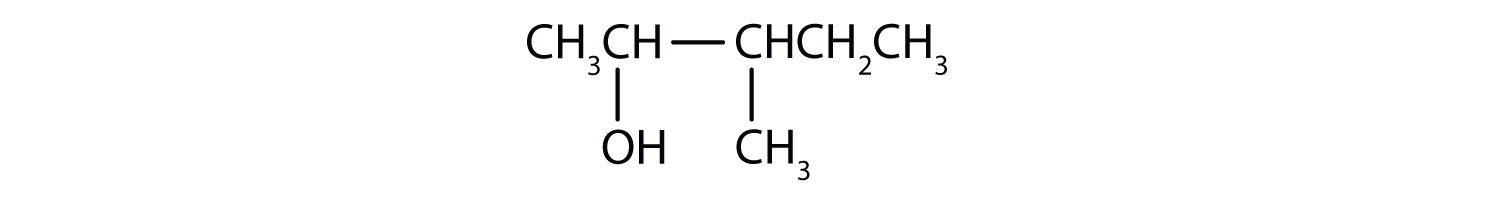
- 3-methyl-two-pentanol
Solution
- The ending -ol indicates an alcohol (the OH functional group), and the hex- stem tells us that there are six carbon atoms in the LCC. We start by drawing a chain of six carbon atoms: –C–C–C–C–C–C–.
The 2 indicates that the OH group is attached to the second carbon cantlet.
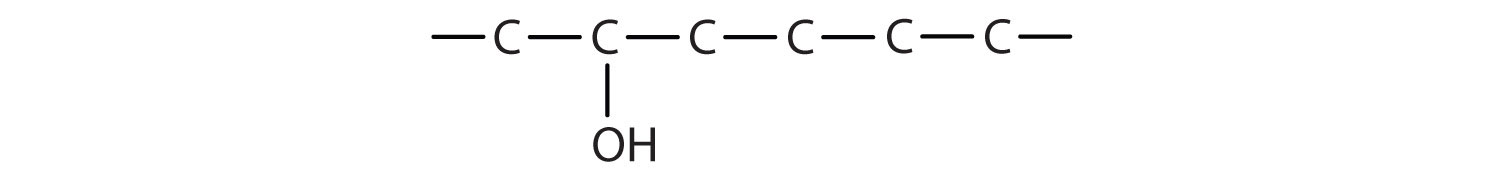
Finally, nosotros add enough hydrogen atoms to give each carbon cantlet four bonds.
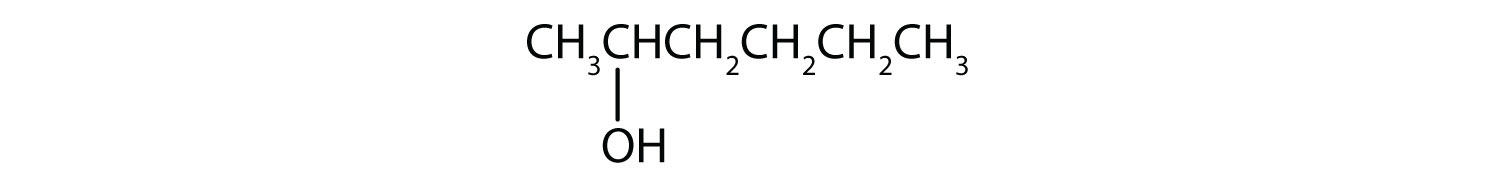
- The ending -ol indicates an OH functional group, and the pent- stem tells the states that there are five carbon atoms in the LCC. We get-go by cartoon a concatenation of 5 carbon atoms: –C–C–C–C–C–
The numbers indicate that there is a methyl (CH3) group on the third carbon atom and an OH group on the second carbon atom.
Practise \(\PageIndex{2}\)
Depict the construction for each compound.
-
iii-heptanol
-
2-methyl-3-hexanol
Classification of Alcohols
Some of the properties of alcohols depend on the number of carbon atoms attached to the specific carbon cantlet that is attached to the OH group. Alcohols tin be grouped into three classes on this ground.
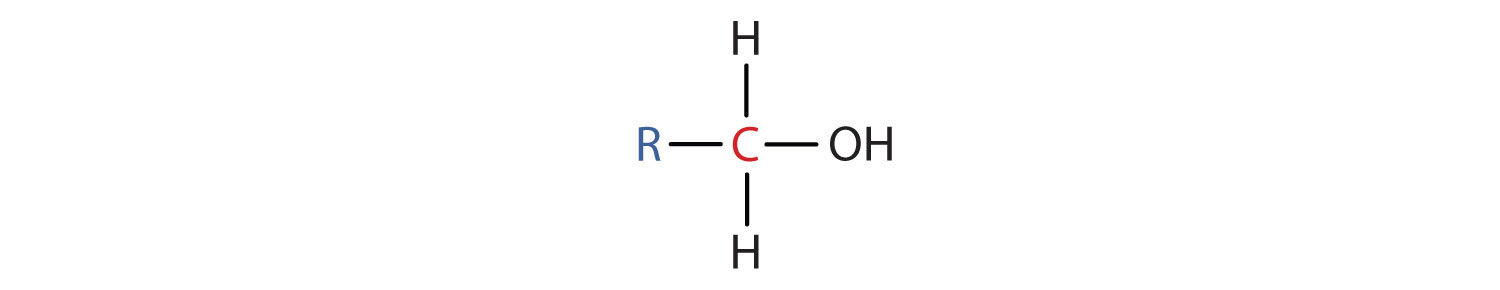
- A primary (one°) alcohol is one in which the carbon atom (in red) with the OH group is attached to ane other carbon atom (in blue). Its general formula is RCH2OH.
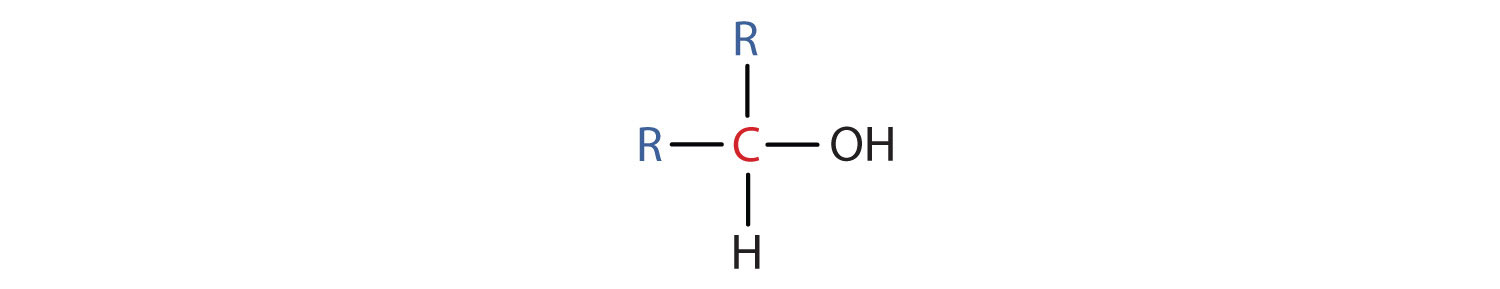
- A secondary (ii°) alcohol is 1 in which the carbon atom (in crimson) with the OH grouping is attached to 2 other carbon atoms (in blue). Its full general formula is R2CHOH.
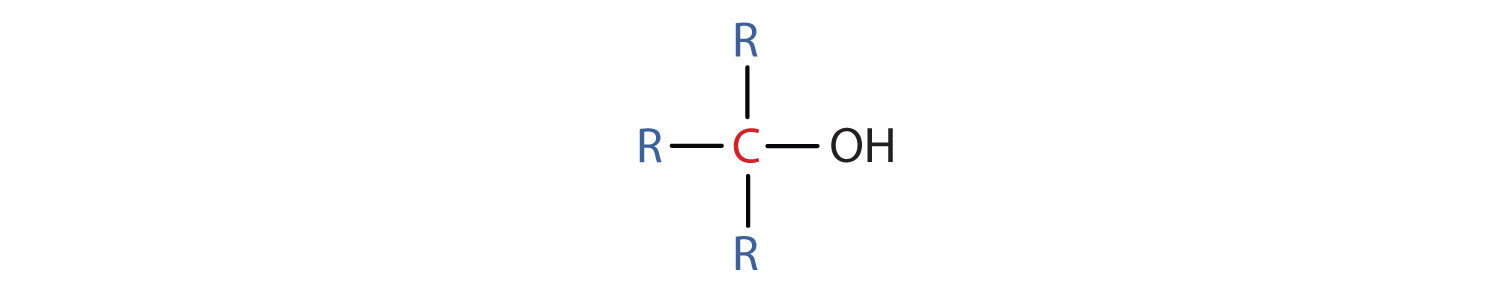
- A third (iii°) booze is 1 in which the carbon atom (in red) with the OH group is attached to three other carbon atoms (in blue). Its general formula is R3COH.
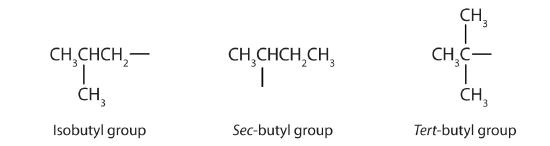
Tabular array \(\PageIndex{1}\) names and classifies some of the simpler alcohols. Some of the common names reverberate a compound's nomenclature as secondary (sec-) or tertiary (tert-). These designations are non used in the IUPAC nomenclature arrangement for alcohols. Notation that there are four butyl alcohols in the table, corresponding to the four butyl groups: the butyl group (CH3CHtwoCH2CH2) discussed before, and three others:
| Condensed Structural Formula | Class of Alcohol | Mutual Name | IUPAC Proper name |
|---|---|---|---|
| CH3OH | — | methyl alcohol | methanol |
| CH3CH2OH | primary | ethyl booze | ethanol |
| CH3CH2CHiiOH | chief | propyl alcohol | one-propanol |
| (CH3)2CHOH | secondary | isopropyl alcohol | two-propanol |
| CH3CH2CH2CH2OH | master | butyl booze | ane-butanol |
| CH3CH2CHOHCHiii | secondary | sec-butyl alcohol | 2-butanol |
| (CH3)2CHCHtwoOH | principal | isobutyl alcohol | 2-methyl-1-propanol |
| (CH3)3COH | tertiary | tert-butyl booze | 2-methyl-2-propanol |
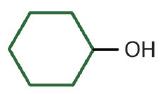 | secondary | cyclohexyl alcohol | cyclohexanol |
Summary
In the IUPAC system, alcohols are named by changing the catastrophe of the parent alkane series proper noun to -ol. Alcohols are classified co-ordinate to the number of carbon atoms attached to the carbon cantlet that is attached to the OH group.
Concept Review Exercises
-
Is isobutyl alcohol primary, secondary, or tertiary? Explain.
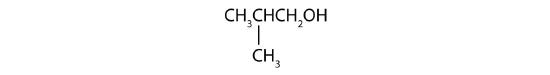
-
What is the LCC in 2-ethyl-1-hexanol? What is taken as the LCC in naming the compound? Explain.
Answers
-
primary; the carbon atom bearing the OH group is attached to only i other carbon atom
-
7 carbon atoms; the six-atom chain includes the carbon atom bearing the OH group
Exercises
-
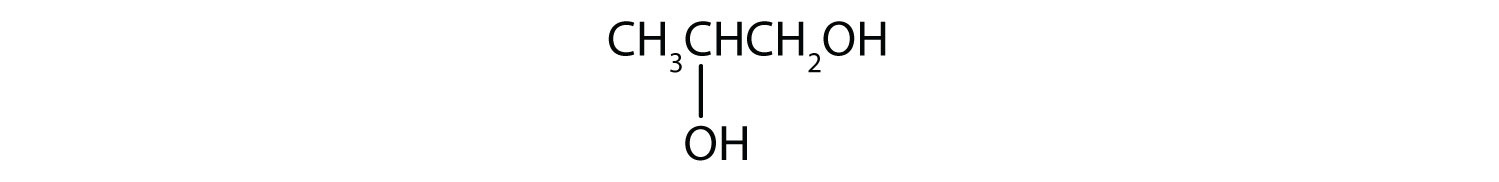
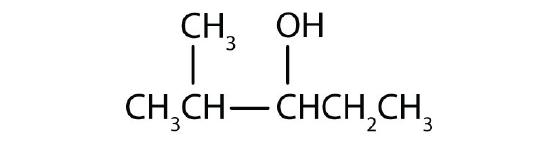
Name each alcohol and allocate it as primary, secondary, or tertiary.
- CH3CH2CH2CH2CH2CH2OH
-
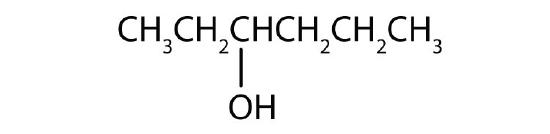
-
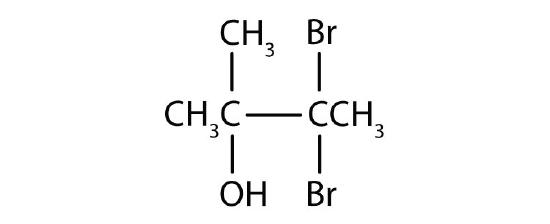
-
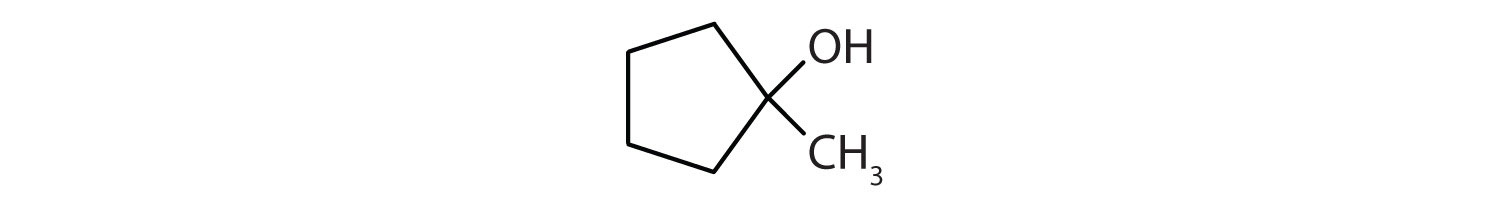
Name each alcohol and allocate it as main, secondary, or 3rd.
-
-
Draw the structure for each booze.
- three-hexanol
- 3,iii-dimethyl-2-butanol
- cyclobutanol
-
Draw the structure for each alcohol.
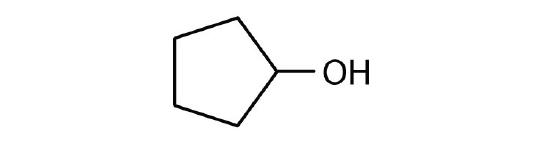
- cyclopentanol
- iv-methyl-2-hexanol
- 4,5-dimethyl-3-heptanol
Answers
-
- i-hexanol; primary
- three-hexanol; secondary
- iii,3-dibromo-ii-methyl-ii-butanol; third
Source: https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/The_Basics_of_GOB_Chemistry_(Ball_et_al.)/14%3A_Organic_Compounds_of_Oxygen/14.02%3A_Alcohols_-_Nomenclature_and_Classification
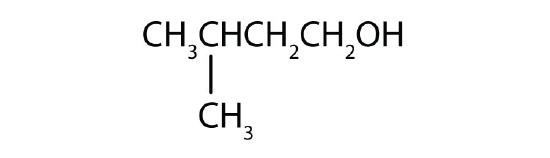
0 Response to "what group of organic compounds has a hydroxyl group -o-h attached to a carbon atom?"
إرسال تعليق